NANOMATERIALS FOR ENERGY STORAGE
Understanding the Role of the Electrolyte in SEI Formation for Li-ion Batteries
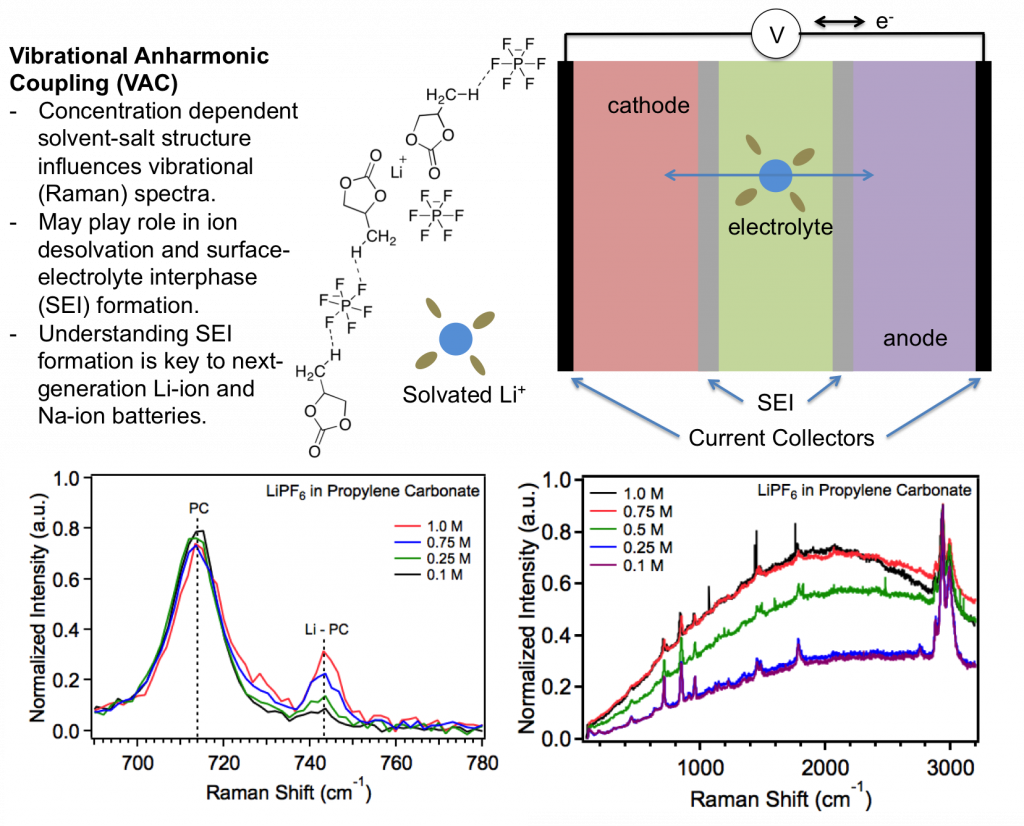 Electrolytes are often viewed simply as a passive transport medium for lithium-ion conduction between the cathode and anode. However, the electrolyte has recently seen a resurgence of research interest due to advances in surface characterization techniques available to study its interactions with electrode materials and also, importantly, because many end-of-life metrics (capital cost and stability) and failure mechanisms (safety) are intimately related to the electrolyte and its interaction with electrode surfaces during charge and discharge. Furthermore, realization of large-scale battery infrastructure will necessitate an understanding of the desired properties and function of electrolytes for next-generation anode and cathode materials.
Electrolytes are often viewed simply as a passive transport medium for lithium-ion conduction between the cathode and anode. However, the electrolyte has recently seen a resurgence of research interest due to advances in surface characterization techniques available to study its interactions with electrode materials and also, importantly, because many end-of-life metrics (capital cost and stability) and failure mechanisms (safety) are intimately related to the electrolyte and its interaction with electrode surfaces during charge and discharge. Furthermore, realization of large-scale battery infrastructure will necessitate an understanding of the desired properties and function of electrolytes for next-generation anode and cathode materials.
Students involved in this project: Anthony Dylla, Logan Bishop
Stability of High Voltage Phosphate Cathodes in Non-aqueous Electrolytes
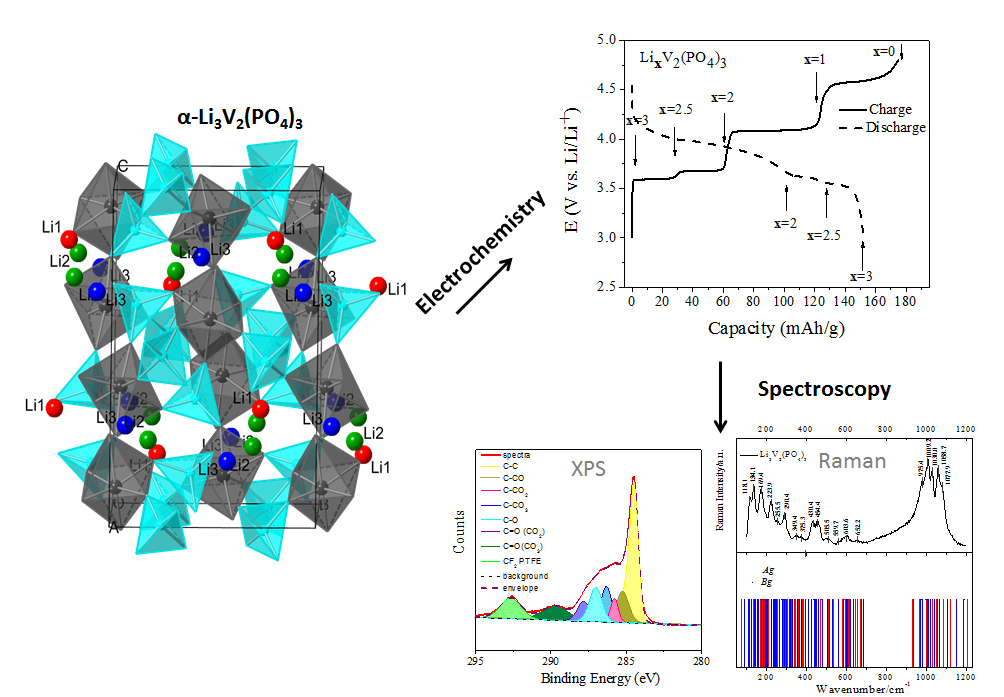
Simple oxide cathodes in lithium-ion batteries (LIBs) offer high voltages but lack the safety and stability for large scale applications (ex: plug-in hybrid electric vehicles). By replacing the O2- ions with XO4n- polyanions it’s possible to tune the position of redox levels in solids and have higher cell voltages. However, these high voltage cathodes operate outside of the electrochemical stability window for the common electrolytes (ex: LiPF6 in organic carbonates). Additionally, LiPF6 is known to dissociate and in the presence of moisture forms several products including HF which can corrode the electrode [114]. Here we present Li3V2(PO4)3 as a model high voltage phosphate cathode and study the surface reactions that take place with the electrolyte to better understand its degradation mechanism. Moreover, we are interested in detecting the charge transfer properties of this material via in situ Raman [123].
Students involved in this project: Nellymar Membreno
Spectroelectrochemical Imaging of the Lithium Alloying Reaction
 By employing both conventional and non-conventional nano- and micro-patterning techniques in conjunction with chemical and electrochemical deposition methods, we have been able to fabricate composite assemblies that are useful as 1D and 2D optical transmission gratings in chemical sensing applications. We have recently developed a new method to study the lithium insertion reaction in metal oxides and mixed metal oxides through diffraction based spectroelectrochemical imaging. This method involves microscope-CCD detection and monitoring the influence of chemically- and electrochemically-responsive diffraction from 1D redox-active transition-metal oxide gratings [61]. This system offers improvements in precision, signal-to-noise ratio, and sensitivity of diffraction-based sensing measurements due to improved spatial resolution, speed of data acquisition and selective wavelength tuning. Current studies are exploring the lithium alloying reaction of colloidal assemblies and arrays of Si nanospheres in order to understand volume expansion/contraction processes and reveal factors that govern charge transfer reactivity.
By employing both conventional and non-conventional nano- and micro-patterning techniques in conjunction with chemical and electrochemical deposition methods, we have been able to fabricate composite assemblies that are useful as 1D and 2D optical transmission gratings in chemical sensing applications. We have recently developed a new method to study the lithium insertion reaction in metal oxides and mixed metal oxides through diffraction based spectroelectrochemical imaging. This method involves microscope-CCD detection and monitoring the influence of chemically- and electrochemically-responsive diffraction from 1D redox-active transition-metal oxide gratings [61]. This system offers improvements in precision, signal-to-noise ratio, and sensitivity of diffraction-based sensing measurements due to improved spatial resolution, speed of data acquisition and selective wavelength tuning. Current studies are exploring the lithium alloying reaction of colloidal assemblies and arrays of Si nanospheres in order to understand volume expansion/contraction processes and reveal factors that govern charge transfer reactivity.
Students involved in this project: Jonathon Duay
SEI Formation – Measurements in Situ and in Anoxic Conditions
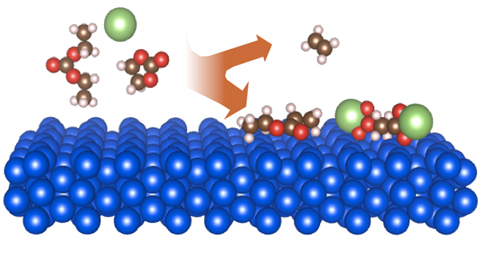 Silicon is a promising next generation negative electrode material to increase energy density in lithium-ion batteries. However, a solid-electrolyte interphase (SEI) forms on the surface of silicon when it is cycled in common commercial electrolytes (LiPF6 in carbonate electrolytes). SEI consists of reduced electrolyte products (e.g., esters, ethers, carboxylates) at the surface of silicon materials, formed by a complex process of multiple competing reaction mechanisms. The resulting SEI is an electronically and ionically insulting film that is highly sensitive to ambient conditions. In order to better understand the electric potential dependence of the SEI formation reactions, species composition, and stability over time, we have developed in situ and anoxic characterization techniques. Currently, Ellipsometry, XPS, ToF-SIMS and FTIR spectroscopy are being used to probe SEI structure, chemical composition, and transport properties as it forms at varying potentials.
Silicon is a promising next generation negative electrode material to increase energy density in lithium-ion batteries. However, a solid-electrolyte interphase (SEI) forms on the surface of silicon when it is cycled in common commercial electrolytes (LiPF6 in carbonate electrolytes). SEI consists of reduced electrolyte products (e.g., esters, ethers, carboxylates) at the surface of silicon materials, formed by a complex process of multiple competing reaction mechanisms. The resulting SEI is an electronically and ionically insulting film that is highly sensitive to ambient conditions. In order to better understand the electric potential dependence of the SEI formation reactions, species composition, and stability over time, we have developed in situ and anoxic characterization techniques. Currently, Ellipsometry, XPS, ToF-SIMS and FTIR spectroscopy are being used to probe SEI structure, chemical composition, and transport properties as it forms at varying potentials.
Students involved in this project: Kjell Schroder
Model Electrodes for High-Resolution Interfacial Charge Transfer Studies
Many of the projects in the Stevenson group address understanding charge transfer mechanisms in various electrochemical energy storage materials, such as lithium ion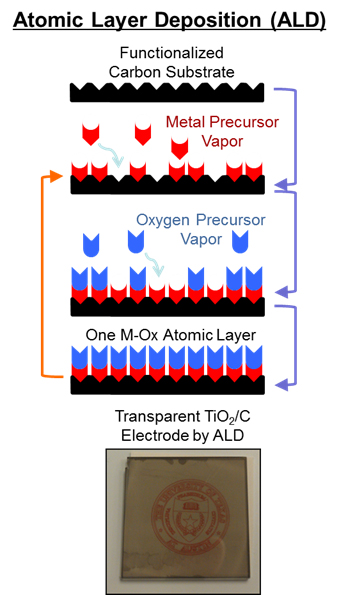 battery electrodes. One approach to study such processes is by engineering materials systems specifically designed to facilitate high-resolution analytical techniques. In order to study the lithium ion-coupled electron transfer processes at the interface between electrodes and the electrolyte in batteries, TiO2 and V2O5 planar electrodes are being developed via atomic layer deposition (ALD). As a result of the inherently precise command of the chemical, structural, and geometric properties exhibited by ALD, it is an ideal synthesis method for creating model materials interface systems. The ability to control the formation of these metal oxide electrodes on a sub-nanometer scale has enabled previously unused analytical tools to be used to investigate these charge transfer processes, including optical absorption techniques and three-dimensionally resolved surface analytical methods. In addition to Li-based energy storage, these methods also show potential for similar interfacial charge transfer studies in organic-inorganic photovoltaics and redox pseudocapacitance materials.
battery electrodes. One approach to study such processes is by engineering materials systems specifically designed to facilitate high-resolution analytical techniques. In order to study the lithium ion-coupled electron transfer processes at the interface between electrodes and the electrolyte in batteries, TiO2 and V2O5 planar electrodes are being developed via atomic layer deposition (ALD). As a result of the inherently precise command of the chemical, structural, and geometric properties exhibited by ALD, it is an ideal synthesis method for creating model materials interface systems. The ability to control the formation of these metal oxide electrodes on a sub-nanometer scale has enabled previously unused analytical tools to be used to investigate these charge transfer processes, including optical absorption techniques and three-dimensionally resolved surface analytical methods. In addition to Li-based energy storage, these methods also show potential for similar interfacial charge transfer studies in organic-inorganic photovoltaics and redox pseudocapacitance materials.
Students involved in this project: Matthew Charlton
Oxygen Vacancy Mediated Charge Storage and Catalysis in Metal Oxides
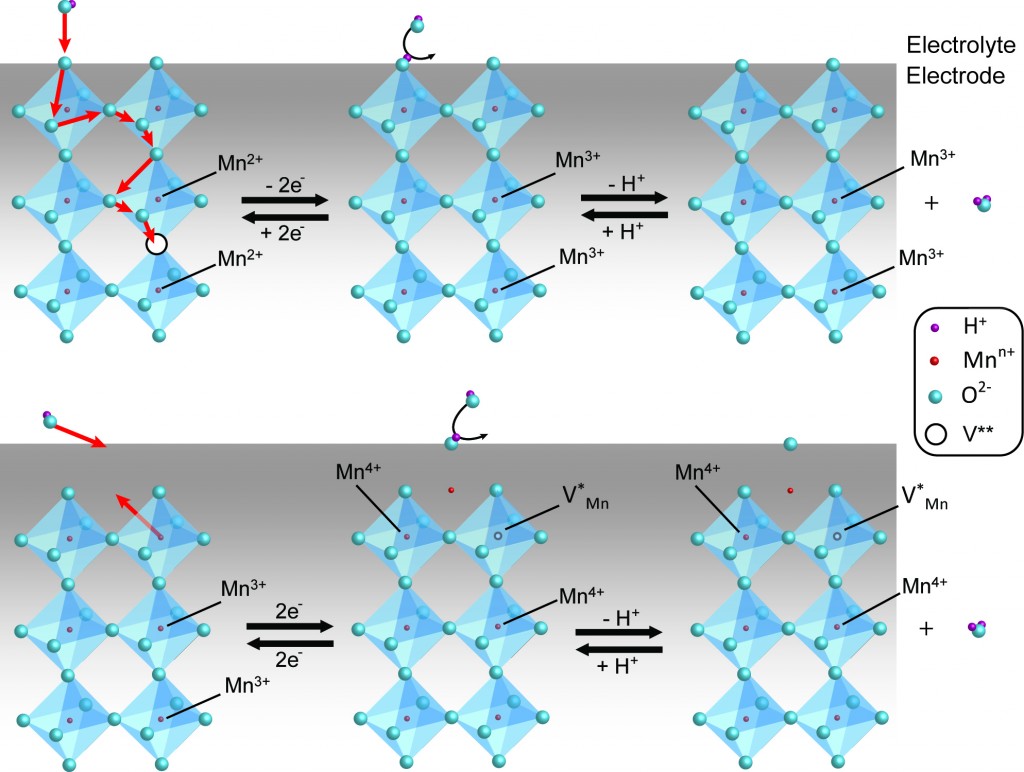 Perovskite oxides have attracted significant attention as energy conversion materials for metal-air battery and solid oxide fuel cell (SOFC) electrodes due to their unique physical and electronic properties. Amongst these unique properties is the structural stability of the cation array in perovskites which can accommodate mobile oxygen ions under electrical polarization. However, in general this phenomenon has been limited to high temperature applications (notably SOFC’s) due to the use of solid state synthetic strategies which result in micron-sized particles. Using nanoscale perovskites, we have demonstrated enhanced diffusion rates of crystal oxygen ions. We are exploring the use of vacancies and mobile oxygen as they relate to electrocatalysis and for pseudocapacitive charge storage [127].
Perovskite oxides have attracted significant attention as energy conversion materials for metal-air battery and solid oxide fuel cell (SOFC) electrodes due to their unique physical and electronic properties. Amongst these unique properties is the structural stability of the cation array in perovskites which can accommodate mobile oxygen ions under electrical polarization. However, in general this phenomenon has been limited to high temperature applications (notably SOFC’s) due to the use of solid state synthetic strategies which result in micron-sized particles. Using nanoscale perovskites, we have demonstrated enhanced diffusion rates of crystal oxygen ions. We are exploring the use of vacancies and mobile oxygen as they relate to electrocatalysis and for pseudocapacitive charge storage [127].
Students involved in this project: Tyler Mefford, Will Hardin, Robin Forslund
NANOMATERIALS FOR ENERGY CONVERSION
Non-Precious Metal Nanoparticles for Bifunctional Oxygen Catalysis
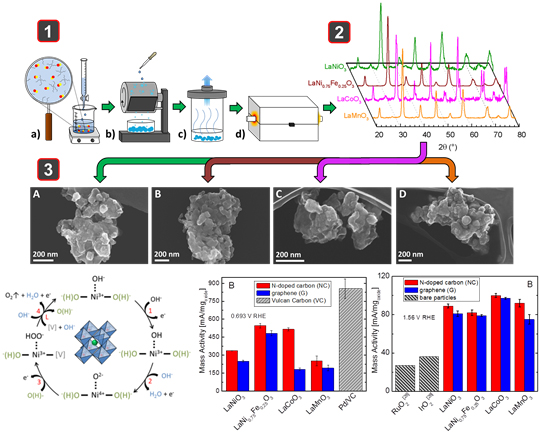 Bifunctional catalysts are able to perform dual roles of reducing oxygen to water and oxidizing water to oxygen, which although nominally appear to be the same reaction, proceed by fundamentally different mechanisms. Traditionally these reactions have been carried out in acidic environments where the reduction of oxygen (ORR) and the oxidation of water (OER) have been dependent on the use of precious metal compounds, such as Pt, Ru, and Ir. In contrast, alkaline environments allow the use of metal oxide systems, leading to the development of non-precious metal materials. We have focused on the development of nanoscale perovskite-type oxides, characterized by the formula ABO3, because of the facility to control the electronic structure of the material through cation doping in both the A and B sites, and through vacancy concentrations in oxygen ion sites. By varying the transition metal in the B-site (B = Ni, Co, Mn, Ni0.75Fe0.25), we have been able to achieve exceptional activities towards both the ORR and OER, leading to materials with better bifunctional character than precious metal systems [116, 135]. We study the activities of these compounds through a variety of analytical techniques, including XPS, XRD, SEM, BET, thin-film rotating disk electrochemistry, and iodometric titrations to learn more about the electronic and physical structures of the materials. Using this knowledge we have begun to develop activity descriptors for perovskites related to vacancy effects and their role in catalyzing intermediates used in the ORR and OER.
Bifunctional catalysts are able to perform dual roles of reducing oxygen to water and oxidizing water to oxygen, which although nominally appear to be the same reaction, proceed by fundamentally different mechanisms. Traditionally these reactions have been carried out in acidic environments where the reduction of oxygen (ORR) and the oxidation of water (OER) have been dependent on the use of precious metal compounds, such as Pt, Ru, and Ir. In contrast, alkaline environments allow the use of metal oxide systems, leading to the development of non-precious metal materials. We have focused on the development of nanoscale perovskite-type oxides, characterized by the formula ABO3, because of the facility to control the electronic structure of the material through cation doping in both the A and B sites, and through vacancy concentrations in oxygen ion sites. By varying the transition metal in the B-site (B = Ni, Co, Mn, Ni0.75Fe0.25), we have been able to achieve exceptional activities towards both the ORR and OER, leading to materials with better bifunctional character than precious metal systems [116, 135]. We study the activities of these compounds through a variety of analytical techniques, including XPS, XRD, SEM, BET, thin-film rotating disk electrochemistry, and iodometric titrations to learn more about the electronic and physical structures of the materials. Using this knowledge we have begun to develop activity descriptors for perovskites related to vacancy effects and their role in catalyzing intermediates used in the ORR and OER.
Students involved in this project: Will Hardin, Tyler Mefford, Robin Forslund
Sub-molecular Atomic Imaging through Kelvin Probe Force Microscopy
Scanning probe microscopy is a powerful tool for studying the real-space morphology and the electronic structure of surfaces and interfaces. Scanning tunneling microscopy (STM) allows real-space imaging of electronic states, as well as spectroscopic characterization of the local density of states. Recent advances in non-contact atomic force microscopy (NC-AFM) and related Kelvin probe force microscopy (KPFM) allow the resolution of individual chemical bonds within molecules, as well as measurements of the local surface electrical potential with submolecular resolution. These capabilities are combined in our Omicron low-temperature STM system.
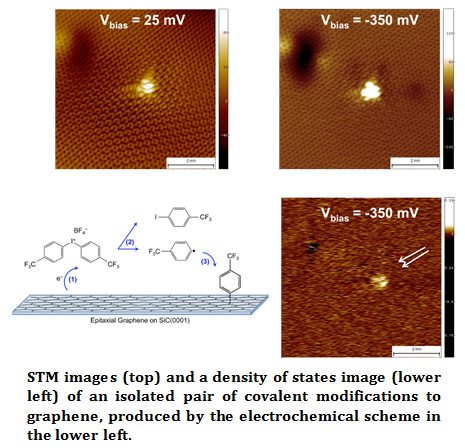 This microscope is used to explore energy-related systems and phenomenon, such as charge separation between donor and acceptor molecules relevant to photovoltaic applications, and the structure of covalent modifications to single layer graphene. In the example shown, an iodonium salt is electrochemically cleaved, resulting in free radicals that reacts rapidly with the single-layer graphene electrode [107]. The STM images show that at energies near the Fermi level, there is extensive scattering of electrons by the modification (~10nm), whereas the states well below the Fermi level show much less scattering and the unperturbed graphene lattice is observed within several nanometers of the modification. The local density of states map clearly resolves the location of the two molecular modifications. Combined with theory and other characterization techniques, scanning probe techniques yield a greater understanding of these energy-related systems.
This microscope is used to explore energy-related systems and phenomenon, such as charge separation between donor and acceptor molecules relevant to photovoltaic applications, and the structure of covalent modifications to single layer graphene. In the example shown, an iodonium salt is electrochemically cleaved, resulting in free radicals that reacts rapidly with the single-layer graphene electrode [107]. The STM images show that at energies near the Fermi level, there is extensive scattering of electrons by the modification (~10nm), whereas the states well below the Fermi level show much less scattering and the unperturbed graphene lattice is observed within several nanometers of the modification. The local density of states map clearly resolves the location of the two molecular modifications. Combined with theory and other characterization techniques, scanning probe techniques yield a greater understanding of these energy-related systems.
Students involved in this project: Alexander Veneman
Electrochemistry in Ionic Liquids
 Ionic liquids have recently gained interest for use as solvents in electrochemistry. These are compounds composed solely of ions that are liquid at room temperature. They offer
Ionic liquids have recently gained interest for use as solvents in electrochemistry. These are compounds composed solely of ions that are liquid at room temperature. They offer
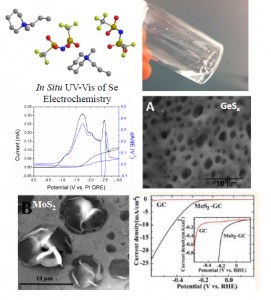
several advantages over traditional aqueous and non-aqueous solvents, such as large electrochemical windows (~4–7 V), air- and water-stable, low volatility and high thermal stability. The properties of ionic liquids can be tuned by changing or modifying the cation or anion, which leads to millions of possible ionic liquids. People have been considering ionic liquids for use in electrodeposition, Li-ion batteries, and electric double layer capacitors. Understanding the fundamental electrochemical and physicochemical properties is essential to developing ionic liquids for use in electrochemical processes and devices. Recently, the Stevenson group has been interested in using ionic liquids to electrodeposit materials that are difficult or impossible to electrodeposit in traditional solvents. We have developed a method to electrodeposit metal sulfides using 1,4-butanedithiol and a metal precursor. GeSx and MoSx have been made by this method [99, 128]. The molybdenum sulfide was found to exhibit interesting photoluminescence properties and high activity for the hydrogen evolution reaction. New and interesting morphologies are possible by adjusting the deposition parameters. We have also been looking at methods to electrodeposit metal selenides, starting with the electrodeposition of elemental Se [132]. In addition, we have developed and characterized new ionic liquids that have large electrochemical windows, ~7V, which could be useful for electrodeposition and high temperature batteries [124].
Students involved in this project: Daniel Redman.
NANOMATERIALS FOR ELECTROANALYSIS
Electrochemistry and Bioelectrochemistry with Carbon Nanomaterials
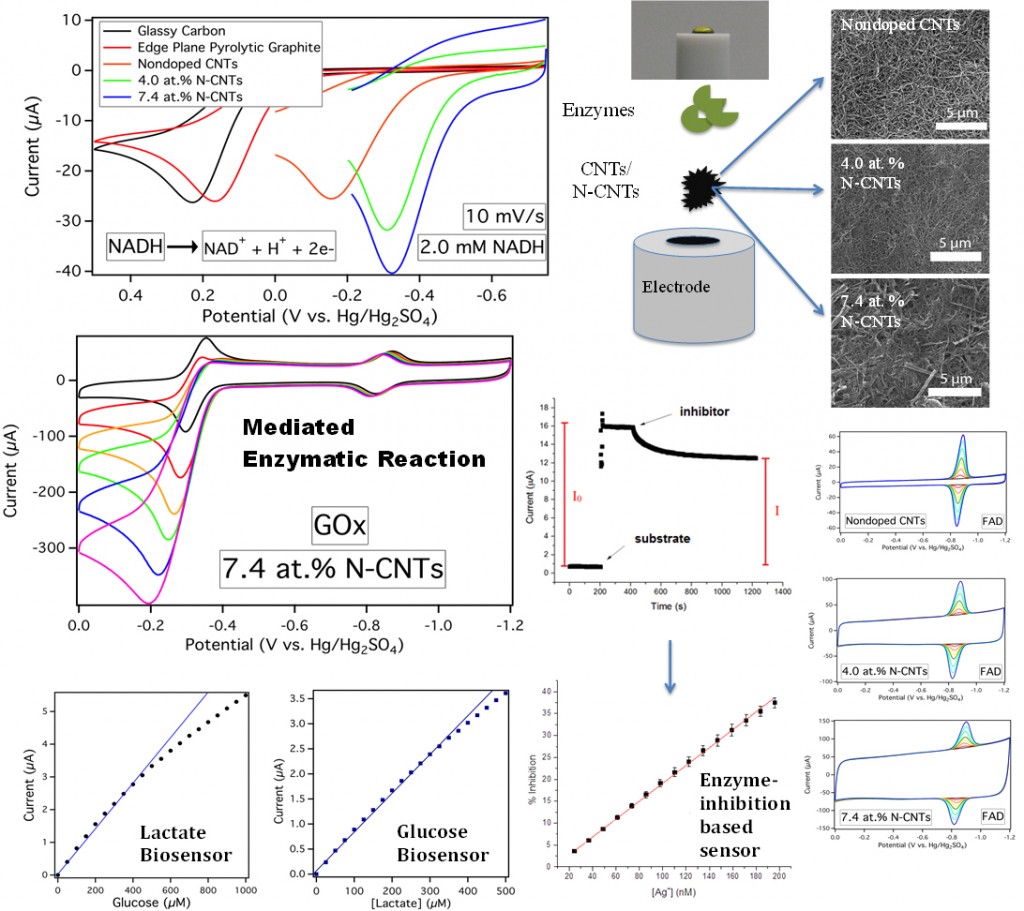 Carbon nanomaterials such as carbon nanotubes (CNTs) display excellent physiochemical properties including high mechanical strength, chemical stability, and a large surface to volume ratio. As an electrode material, CNTs display rapid electrode kinetics, minimal surface fouling, and low overvoltages for many electrochemical reactions. Doped CNTs, and nitrogen doped CNTs (N-CNTs) in particular, offer a unique way to alter the inherent properties of CNTs due to the incorporation of nitrogen into the carbon lattice [27,32,35,36,67]. Our group has previously shown that N-CNTs catalyze oxygen reduction via a peroxide pathway which also catalytically disproportionates H2O2 [32,85]. The fast disproportionation of H2O2 provides a unique method to electrochemically detect analytes using H2O2 producing enzymes [63,89]. Current projects focus on the utilization of enzymatic inhibition to attenuate electrode-immobilized enzymatic activity, effectively creating a highly-sensitive sensing scheme for enzyme inhibitors, such as heavy metals. In addition to this, we also investigate the electrochemical behavior of enzymes and their cofactors, such as NADH [126] and FAD[117, 131], at CNT/N-CNT electrodes. These studies should help the guide the development of bioelectronics, biosensors, and biofuel cells.
Carbon nanomaterials such as carbon nanotubes (CNTs) display excellent physiochemical properties including high mechanical strength, chemical stability, and a large surface to volume ratio. As an electrode material, CNTs display rapid electrode kinetics, minimal surface fouling, and low overvoltages for many electrochemical reactions. Doped CNTs, and nitrogen doped CNTs (N-CNTs) in particular, offer a unique way to alter the inherent properties of CNTs due to the incorporation of nitrogen into the carbon lattice [27,32,35,36,67]. Our group has previously shown that N-CNTs catalyze oxygen reduction via a peroxide pathway which also catalytically disproportionates H2O2 [32,85]. The fast disproportionation of H2O2 provides a unique method to electrochemically detect analytes using H2O2 producing enzymes [63,89]. Current projects focus on the utilization of enzymatic inhibition to attenuate electrode-immobilized enzymatic activity, effectively creating a highly-sensitive sensing scheme for enzyme inhibitors, such as heavy metals. In addition to this, we also investigate the electrochemical behavior of enzymes and their cofactors, such as NADH [126] and FAD[117, 131], at CNT/N-CNT electrodes. These studies should help the guide the development of bioelectronics, biosensors, and biofuel cells.
Students involved in this project: Ian Rust, Mi Chen, Janine Elliott, Ethan Phan
Single Collision Electrochemical Analysis of Nanoparticles
 The increasing interest in nanoparticles (NPs) due to their unique properties and
The increasing interest in nanoparticles (NPs) due to their unique properties and
multifarious applications has necessitated the development of new analytical tools for characterizing NPs in a fast and reproducible manner. Especially, to better understand the fundamental properties, correlate structure-function relationships of NPs and optimize NPs activity for various applications analytical tools that enable precise characterization of individual NPs in terms of their size, shape and composition are of immense importance. The development of “electrocatalytic amplification” is one such method where single NPs are detected by measuring electrocatalytic current due to the electrochemical processes (oxidation/reduction of the species present in solution) occurring on the surface of the NP whenever a NP collides with an inert UME (gold, platinum, platinum oxide and carbon) which otherwise cannot catalyze the reaction.
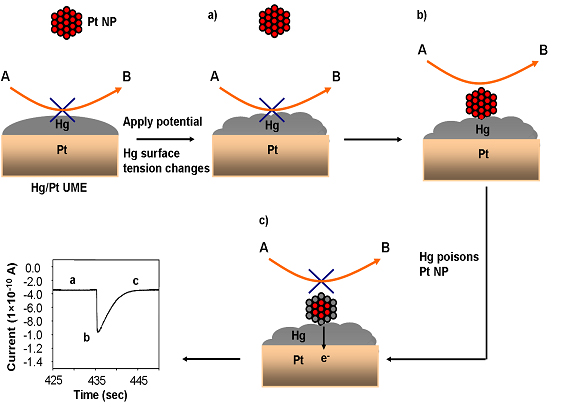
We recently reported the advantages of utilizing Hg/Pt UME as an electrode platform for detecting and screening Pt NP sizes through electrocatalytic amplification using hydrazine oxidation as the redox indicator reaction [118,129]. Figure 1. shows the schematic representation of the principle involved in single Pt NP detection at Hg/Pt UMEs. Single NPs were detected through an increase in current caused due to the electrocatalytic reaction (hydrazine oxidation) occurring at the surface of the NP when Pt NP contacts a Hg/Pt UME. Once the NP contacts the Hg /Pt electrode, Hg poisons the Pt NP and deactivates the electrocatalytic reaction. Hence, at Hg/Pt UMEs, the i-t response consists of repeated current “spikes” that return to the background level as Hg poisons the Pt NP after collision with the Hg/Pt UME due to amalgamation and deactivation of the redox reaction. In contrast a stepwise “staircase” response was previously described at a Au UME under similar conditions as the NP retains its catalytic activity after the NP is stuck to the Au UME. Size distributions for NP ranging in size from ca. 4 to 24 nm determined from the i-t transients correlated well with theory and TEM derived size distributions. The use of Hg as an electrode material has several quantitative advantages including suppression of the background current by two orders of magnitude over a Au UME; increased signal to noise (S/N) for detection of individual collisions; precise integration of current transients to determine charge passed and NP size; reduction of surface induced NP aggregation and electrode fouling processes; and reproducible and renewable electrodes for routine detection of catalytic NPs. Furthermore, at a Hg/Pt UME the applied potential directly influences the interfacial surface tension (electrocapillarity) which also impacts the observed i-t response for single Pt NP collisions for proton reduction which exhibits a faster decay of current (0.7 to 4 ms) to background levels compared to hydrazine oxidation (2 to 5 s) [129]. Since the surface tension of Hg is lower at -0.9 V vs. Ag/AgCl, Pt NPs possibly react faster with Hg (amalgamate at a faster rate) resulting in sharp current “spikes” for proton reduction compared to hydrazine oxidation (-0.05 V). Also, potentiometric method seems to be more sensitive than amperometric method for detecting single Pt NPs [137]. In potentiometric method single Pt NPs are detected by measuring the changes inopen circuit potential (OCP) instead of the current (amperometric method) during single Pt NP collisions with the mercury modified Pt ultramicroelectrode (Hg/Pt UME) [137].
We currently are investigating the effect of ligand, morphology and composition of NPs on the current-time response during single NP collisions with Hg/Pt UME.
Students involved in this project: Radhika Dasari, Donald Robinson
Improving the Limit of Detection for Nanoimpact Electroanalysis with Magnetically Guided Bifunctional Catalytic/Superparamagnetic Nanoparticles
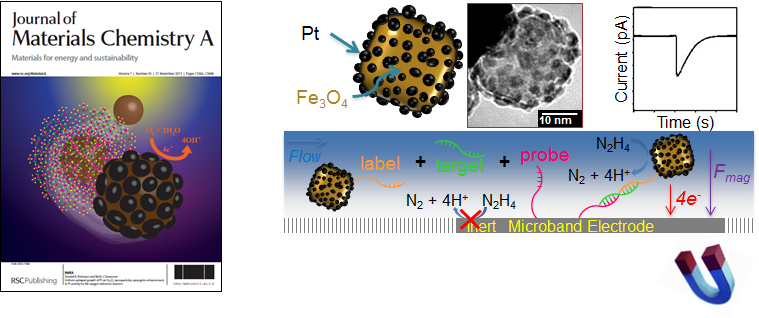 An new technique that is rapidly gaining interest in the field of nanoscale electrochemistry involves the detection of individual nanoparticles from a colloidal suspension by the process of electrocatalytic amplification. The chronoamperometric method relies on three major components: 1) a small molecule electrochemical indicator species, 2) nanoparticles that are catalytically active for driving the reduction or oxidation of the indicator species, and 3) an ultramicroelectrode inert to the indicator species. When a catalytic nanoparticle comes into contact with the electrode held at an appropriate potential bias, single-nanoparticle electrocatalysis occurs to produce discreet transient current signals. We are working together with collaborators in an outside of UT-Austin to develop this electrochemical method into a new form of nanotechnology for ultrasensitive lab-on-a-chip electrochemical biosensors. While the sensitivity of nanoimpact methods can be considered superior due to single-particle signal response, the practical limit of detection still suffers if left to rely on the slow diffusion of nanoparticles to a detection platform of microscale dimensions. We have therefore developed methods to magnetophoretically drive bifunctional catalytic/superparamagnetic nanoparticles to the electrode surface and maximize the frequency of observed nanoparticle@electrode collisions. The strategy relies on Pt-decorated iron oxide nanoparticles which were synthesized by controlled growth of Pt on amine-functionalized iron oxide core nanoparticles [130]. The application of a tightly focused external magnetic field to the ultramicroelectrode leads to a substantial increase the relative frequency of chronoamperometric signals corresponding to discrete nanoparticle adsorption events. We are working to further optimize this system for achieving ultratrace limits of nanoparticle detection. With proper biomodification, we hope to develop the approach into a general affinity-based sensing platform for detecting various target biomolecules at subattomolar concentrations.
An new technique that is rapidly gaining interest in the field of nanoscale electrochemistry involves the detection of individual nanoparticles from a colloidal suspension by the process of electrocatalytic amplification. The chronoamperometric method relies on three major components: 1) a small molecule electrochemical indicator species, 2) nanoparticles that are catalytically active for driving the reduction or oxidation of the indicator species, and 3) an ultramicroelectrode inert to the indicator species. When a catalytic nanoparticle comes into contact with the electrode held at an appropriate potential bias, single-nanoparticle electrocatalysis occurs to produce discreet transient current signals. We are working together with collaborators in an outside of UT-Austin to develop this electrochemical method into a new form of nanotechnology for ultrasensitive lab-on-a-chip electrochemical biosensors. While the sensitivity of nanoimpact methods can be considered superior due to single-particle signal response, the practical limit of detection still suffers if left to rely on the slow diffusion of nanoparticles to a detection platform of microscale dimensions. We have therefore developed methods to magnetophoretically drive bifunctional catalytic/superparamagnetic nanoparticles to the electrode surface and maximize the frequency of observed nanoparticle@electrode collisions. The strategy relies on Pt-decorated iron oxide nanoparticles which were synthesized by controlled growth of Pt on amine-functionalized iron oxide core nanoparticles [130]. The application of a tightly focused external magnetic field to the ultramicroelectrode leads to a substantial increase the relative frequency of chronoamperometric signals corresponding to discrete nanoparticle adsorption events. We are working to further optimize this system for achieving ultratrace limits of nanoparticle detection. With proper biomodification, we hope to develop the approach into a general affinity-based sensing platform for detecting various target biomolecules at subattomolar concentrations.
Students involved in this project: Donald Robinso, Radhika Dasari
Investigation of Housed Cellular Communities using Electrochemical Nanoprobes
Eukaryotes consist of membrane enclosed organelles and can exist as both unicellular and multicellular organisms are vastly more complex than that of their evolutionary counterparts, prokaryotes. Except for few anomalies, prokaryotes exclusively exist as unicellular entities, and can circumstantially organize into biofilms or colonies of independent cells. Although both types of cells structurally differ, they use similar molecular signaling mechanisms to extracellularly communicate within sustained communities.
multicellular organisms are vastly more complex than that of their evolutionary counterparts, prokaryotes. Except for few anomalies, prokaryotes exclusively exist as unicellular entities, and can circumstantially organize into biofilms or colonies of independent cells. Although both types of cells structurally differ, they use similar molecular signaling mechanisms to extracellularly communicate within sustained communities.
The extracellular molecular signaling or “crosstalk” that cells exhibit in vivo can be due to population and density dependent queues. Furthermore, it can be attributed to extracellular chemical, topographical, and mechanical features. The multifaceted environs in which cells interact are highly differentiated, and susceptible to extreme changes encompassing proliferation, and response to toxic foreign bodies/chemicals. Evidence shows that cellular molecular signaling is paramount to the survival and destruction of cellular organisms, yet there is a shortage of methods for systematically and quantitatively assessing the behavior in vitro.
The development and implementation of a highly selective, sensitive, and durable in vitro electrochemical nanoprobe for the localized in vitro detection of extracellular signaling within proliferating clonal cellular colonies. Here, robust novel electrocatalytic nitrogen doped carbon nanotubes (NCNTs) will be integrated into electrospun polymeric fibers to make nano-diameter electrochemical probes (Fig. 2). The probes will be used to make localized in vitro point measurements of clonal cell colonies isolated within 3D protein based hydrogel structures (Fig. 1). The 3D architectures are made using multiphoton photolithographic (MPL or 3D printing) techniques to photocrosslink arbitrary 3 dimensional biocompatible hydrogel architectures around cells, affording new cellular environments (Fig. 3). Fluctuations of the electrochemical measurements combined with optical microscopy could link the electrochemical signal generated from a specific signal molecule to observed cellular behavior in real time.
Students involved in this project: Janine Elliot
Adaptable Ultramicroelectrode Arrays for the Tailored Detection of Analytes
Although reports on the synthesis of microelectrode and ultramicroelectrode arrays in the literature are prevalent, almost all of the fabrication methods require demanding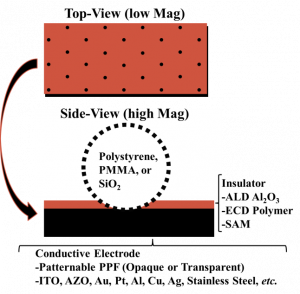 processing steps, expensive equipment, and/or precise instrumentation.
processing steps, expensive equipment, and/or precise instrumentation.
In the Stevenson Lab, we have developed a facile fabrication method that produces ultramicroelectrode arrays that can easily be tailored to the detection of a particular analyte.[141] Fabrication consists of dropcasting suspensions of microspheres (Polystyrene, SiO2, or PMMA) onto a conductive substrate forming a 2-D monolayer. This 2-D network is then coated with an insulating layer using either ALD, insulating polymer electrodeposition, or self-assembled monolayers (SAMs). The spheres are then removed, leaving behind ultramicroelectrodes where the spheres were in contact with the conductive substrate.
The wealth of tunability for this platform allows customization for almost any analyte of interest. Therefore, these arrays can be easily applied to trace analysis in environmental monitoring, as biological sensors, or as detectors for electrophoresis among other applications.
Students involved in this project: Jonathon Duay